Test for Nitrate Ions
Rates of the reaction between i sodium thiosulphate and hydrochloric acid ii potassium iodate. The halide ions can be detected using nitric acid followed by silver nitrate.
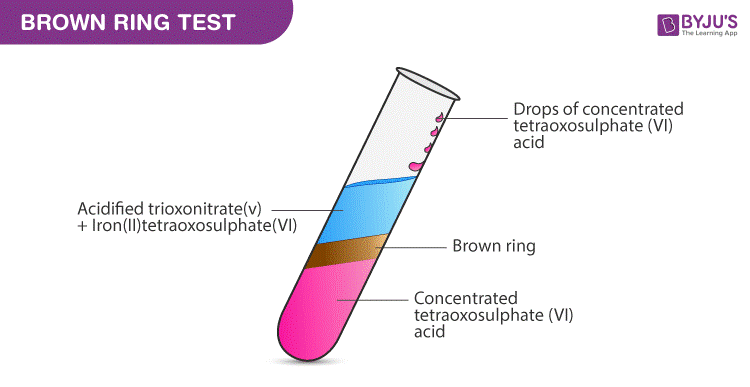
Brown Ring Test Nitrate Test Procedure Reactions On Byju S
On the amount of nitrate in the water supply and the balance of other ions in the water.

. Nitrate ions in. The primary issue at hand is the fact that ammonia can be present in a non-ionized form NH 3 or the ionized form NH 4 known as ammonium. Many proteins in living beings contain bound ironIII ions.
Only ionic compounds which are soluble in water forming aqueous solution will dissociate into ions in water. However arsenate is like phosphate. So for example if you know chlorate you also know bromate and iodate too BrO3- and IO3-.
The nitrate ion carries a formal charge of 1. Observe and record the colour of any precipitate formed. Nitrates are ions NO 3- and fish can gradually adapt to change in the level of ions and ionic compounds such as salts in their environment.
Characteristics should be verified by tests conducted under established test procedures and water analysis. Nearly all living organisms from bacteria to humans store iron as microscopic crystals 3 to 8 nm in. Fluctuating pH levels can also affect the reading by the meter.
However not all ammonia tests are created equal. The body of a freshwater fish contains more ions and ionic compounds than the water. Silver nitrate dilute nitric acid The nitric acid reacts with and removes other ions that might also give a confusing precipitate with silver nitrate.
There are some exceptions. The accuracy of the electrode can be affected by high concentrations of chloride or bicarbonate ions in the sample water. Equilibrium studies involving i ferric and thiocyanate ions ii CoH2O6 and chloride ions.
The reduction of silver ions into metallic silver results in the formation of a silver mirror on. This test has to be done in solution. Similarly the photolysis of DOM in the presence of halide ions can be an alternative and significant pathway to generate reactive oxygen and halogen.
This reacts with chronotropic acid to produce a red-orange complex directly proportional to the amount of nitrite present. The Tollens reagent is the alkaline solution of silver nitrate AgNO 3 mixed with liquid ammonia NH 3. Insoluble substance cannot dissociate into ions in water.
Nitrite which is reduced from nitrate by gastro-intestinal microorganisms oxidizes the hemoglobins ferrous ions into ferric ions and consequently disables the oxygen transport mechanisms of the red blood cells. Ions that end in ate have oxygen in them. The reactive halogen radicals were generated from nitrate photolysis in the presence of naturally abundant halide ions which facilitated the oxidation of Mn 2 aq to δ-MnO 2 nanosheets.
This charge results from a combination formal charge in which each of the three oxygens carries a 2 3 charge whereas the nitrogen carries a 1 charge all these. The common spectrophotometric methods depend on nitrate reduction. For example the Griess-IIosvay method measures the absorbance of nitrite resulting from nitrate reduction reactions Pasquali et al 2007.
Those are an important subclass of the metalloproteinsExamples include oxyhemoglobin ferredoxin and the cytochromes. The solution is acidified by adding dilute nitric acid. The ion is the conjugate base of nitric acid consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement.
Elements in the same family make similar ions. However the sudden drop of Nitrate levels that follows a water change could send your fish into Osmotic Shock. This method is used for wastewater drinking water surface water and process water.
The percentage of water volume you interchange will correspond to the percentage of nitrate being removed from the system. If you exchange 25 of the water in the tank then that would correspond to removing 25 of the Nitrate given that your tap water is completely free of NO 3 ions. Tollens Test is a chemical test used to differentiate reducing sugars from non-reducing sugars also called the silver mirror test.
Nitrate is not like phosphate even though nitrogen and phosphorus are in the same group. Water with Nitrate-Nitrite as N less than 10 mgL is. Chloride ions will produce a white precipitate bromide a cream precipitate and iodide produces a yellow precipitate.
Strong base neutralization reaction ii hydrogen bonding interaction between acetone and chloroform. Almost all known forms of life particularly complex life require iron. The maximum contaminate level for Nitrate-Nitrite as N in drinking water as determined by the EPA is 10 mgL or parts per million ppm.
Enthalpy determination for i strong acid vs. An ammonia test kit is one of the must-haves for every aquarium owner. This water should not be consumed until corrective action is taken.
To safely reduce the nitrate levels perform a couple of 5 water changes every. Nitrate electrodes and meters are expensive compared to field kits that employ the cadmium reduction method. To tell whether an unknown substance contains ironII nitrate or ironIII nitrate add a few drops of sodium hydroxide solution.
The toxic NH 3 is what hobbyists are concerned about but most tests give results for the total of. In this low range nitrite test nitrite ions react with sulfanilic acid to form an intermediate diazonium salt. The determination of these two ions is usually challenging because of interferences from other materials.
Test for halide ions Add a few drops of dilute nitric acid then a few drops of silver nitrate solution. When tested in ZnVS2 full coin cell configuration with cathode mass loading of 16 mg cm2 the electrolyte solution containing the. Investigating transition metal ions.
The ion exchange process for example is sensitive to waters containing high TDS high sulfate and high. The following diagram shows how to write the ionic equation for the reaction of aqueous sodium carbonate with aqueous barium nitrate. Nitrate NO 3-1 US EPA.
Nitrate in drinking water can lead to methemoglobinemia which is caused by nitrite in the human gastro-intestinal tract 910. If you start from a solid it must first be dissolved in pure water. It is very important that any unreacted hydroxide ions are removed completely to give a neutral or acidic solution as silver hydroxide will also.

Question Video Testing For The Presence Of Nitrate Ions Using The Brown Ring Test Nagwa

Qualitative Analysis Identifying Anions O Level Secondary Chemistry Tuition
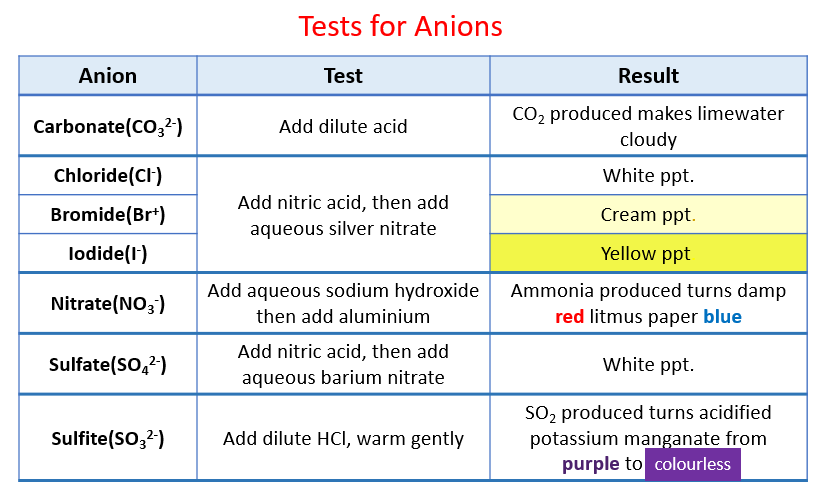
Identify Anions Solutions Examples Activities Experiment Videos

In The Ring Test Of No 3 Ion Fe 2 Ion Reduces Nitrate Ion To Nitric Oxide Which Youtube


Comments
Post a Comment